CAR-T Cell Therapy
Acute lymphoblastic leukemia (ALL) has gained a warrior to help in its fight against cancer. The overall survival rate of B-Cell ALL is very poor (Sun, et. al, 2018). Statistically, children with cancer experience much greater survival rates as compared to adults with cancer (Cancer.gov, 2019). Chimeric Antigen Receptor T cell therapy or CAR-T therapy is an approved immunotherapy treatment for pediatric cancers. Biotherapies and immunotherapies inherently cause fewer side effects than traditional chemotherapy. “Over the past several years, immunotherapy - therapies that enlist and strengthen the power of a patient's immune system to attack tumors—has emerged as what many in the cancer community now call the "fifth pillar" of cancer treatment” (Cancer.gov, 2019).
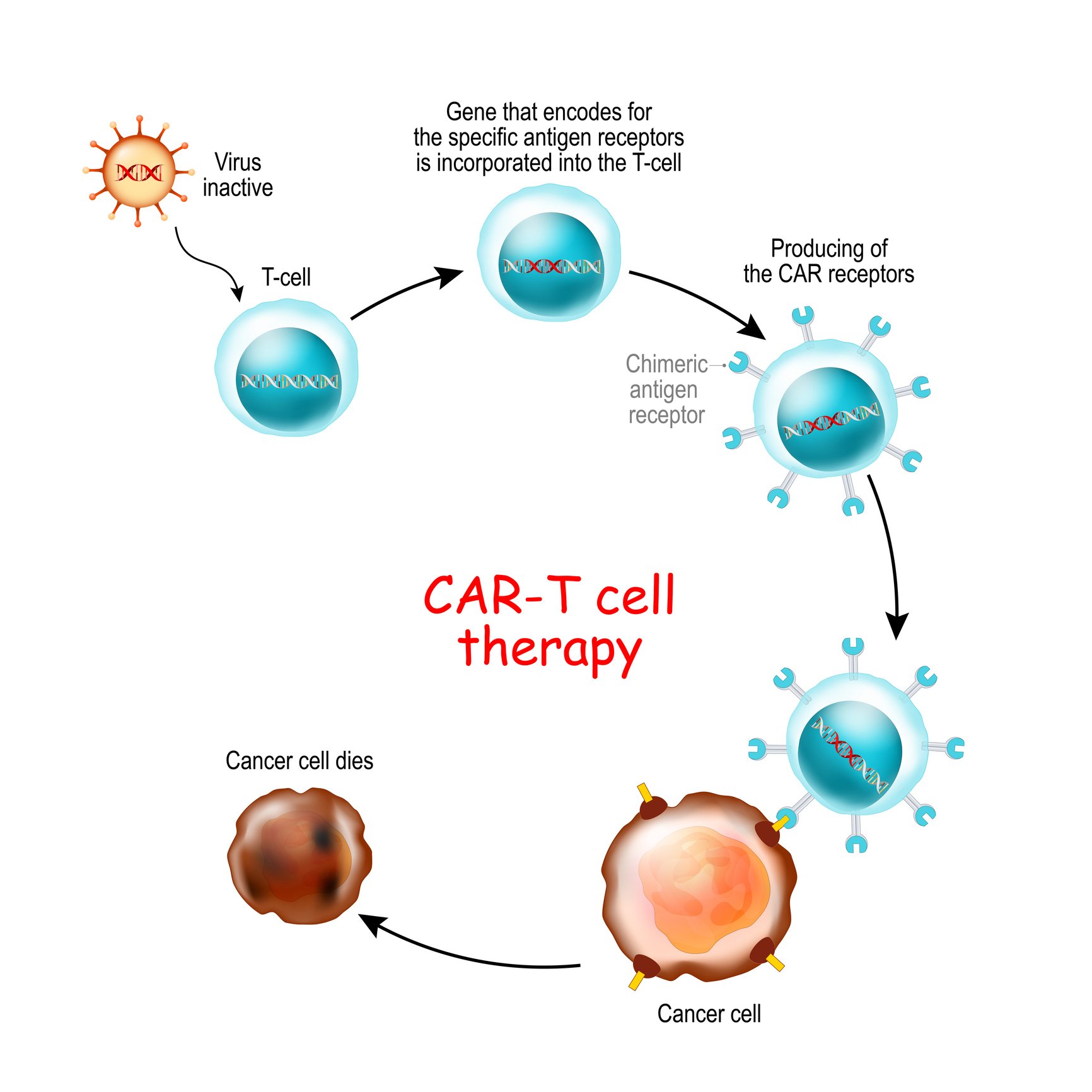
CAR-T therapy is an FDA-approved immunotherapy treatment for refractory B Cell ALL or those patients who have relapsed two or more times. This individualized treatment teaches the patient's very own T cells how to find and kill cancerous cells in the body.
Healthy T cells or Natural Killer (NK) cells are removed from the patient via apheresis. Once the healthy cells are in the lab, they are multiplied and chimeric antigen receptors (CARs) are added to the surface of the T cells. This allows them to easily target the cancerous cells once reinfused into the patient. This large volume of engineered cytotoxic cells is reinfused into the patient, where the cells are able to mount a super-sized attack on their target (Cancer.gov, 2019).
The most common side effects of CAR-T therapy include neurologic toxicities and cytokine release syndrome (CRS) (Cancer.gov, 2019). CRS diagnosis is based on clinical symptoms and treatment is focused on supportive care of presenting symptoms. Because patients that present with CRS often have high levels of interluken 6, it is wise to have the monoclonal antibody available during infusion and for 21 days post-infusion. It can help alleviate CRS symptoms such as inflammation and also decrease circulating cytokines (Sun, et. al, 2018).
Researchers are excited about the possibilities of CAR-T. Other approaches being explored include the development of CAR-T cells with so-called “off switches”. Meaning, that once deemed cancer-free, the T cells stop fighting, thus reducing the side effects such as CRS. Through research, Car-T has become an option for those who are battling ALL.
Research related to CAR-T and its use for other cancerous processes such as lymphomas and solid tumors is ongoing. With time, long-term studies with revealing more information on ways to reduce toxicities and improve the management of the side effects.
References
National Cancer Institute, 2019. CAR T cells: engineering patients’ immune cells to treat their cancers. National Cancer Institute.
Sun, W., Malvar, J., Sposto, R. et al. Outcome of children with multiply relapsed B-cell acute lymphoblastic leukemia: a therapeutic advances in childhood leukemia & lymphoma study. Leukemia 32, 2316–2325 (2018) doi:10.1038/s41375-018-0094-0